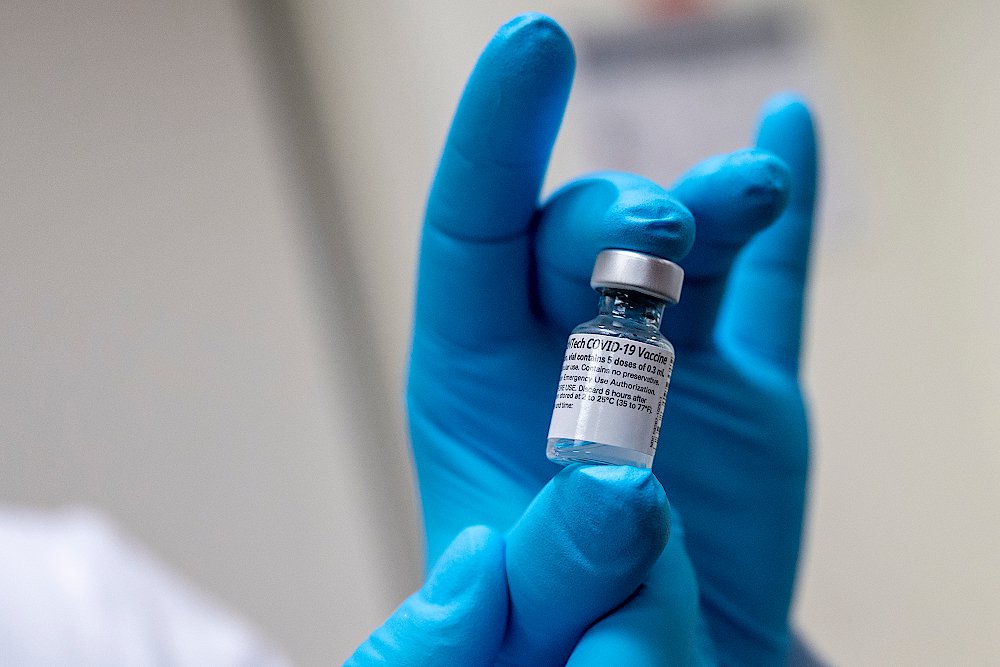
Health chiefs in Brighton are inviting interested members of the public to help trial proposed "booster" anti-coronavirus vaccines.
The formal scientific trial, being helped by the Sussex University Hospitals NHS Foundation Trust, is aimed at finding out how useful additional “booster” vaccinations might be for providing added protection against the coronavirus, above that offered by vaccines already received by the majority of people.
These extra jabs could, if the trials provide positive results for this use, help protect people at higher risk of serious symptoms.
This new study is trying to find out which vaccines against COVID-19 are most effective as a booster vaccination, depending on which vaccine was used to provide the initial prime-boost course.
Scientists running the trials said measurements and data showed that the Oxford/AstraZenca vaccine and the Pfizer/Biontech jabs are highly effective at preventing severe Covid-19 symptoms.
But they added that, at the moment, it's not known how long the immune protection from vaccination will last.
In addition, they said, variants of the virus which causes COVID-19 (SARS-CoV-2) have emerged with mutations which might make the immune response from vaccination less effective.
So it's being considered that additional, “booster” vaccinations might be needed for high risk groups after a period of time to provide added protection.
The scientists said this study is trying to find out which vaccines against COVID-19 are most effective as a booster vaccination, depending on which vaccine was used to provide the initial prime-boost course.
The NHS, through the Brighton and Sussex Universities NHS Foundation Trust, intends to enrol men and women over the age of 30 who received their initial prime-boost course of vaccination against COVID-19 in December 2020 or January 2021.
In particular, the medics are seeking people who are 84 days post second vaccination.
If you wish to take part, you must be willing to tell the trial staff about your medical history, and you may be asked to allow the trial staff to check this with your General Practitioner (GP).
Giving more details about the science behind the trials, the medics said:
"We are studying combinations of seven different COVID-19 vaccines compared to a control group, who will be receive a vaccine against the meningococcal bacteria which causes meningitis and sepsis (Men ACWY).
"There will be 2886 participants.
"As new SARS-CoV-2 vaccines become available, more vaccines may be included in the trial and so the total number of participants may increase (these will be in later stages of the trial).
"Participants will be allocated, at random, (rather like a flip of a coin) to receive one dose of one of the seven study vaccines, or the control vaccine (MenACWY).
"Three of the vaccines are currently approved by the MHRA for NHS use to prevent COVID-19, 2 are under review for approval by the MHRA and 2 are still in clinical trials but may be available in the UK later in 2021 if approved by the MHRA."
They went on to describe exactly what participants could expect, after the trial vaccine is administered.
"Between 4 and 6 routine blood tests will be taken over the course of a year to look at the immune responses to the vaccine depending on the group you are in.
"You may also be asked for a nasal fluid sample and an (optional) saliva sample at each visit.
"You might also be asked to attend for a repeat blood test if there were any safety concerns.
"If you were to test positive for the virus causing COVID-19 we may ask you to attend for an extra visit.
"Participants will need to complete an online diary for up to 28 days following vaccination.
"The trial will take one year to complete per participant (from the time the first dose of vaccine is given)."
As with almost every trial of a treatment, participants would not know which vaccine they had received until the end of the trial.
If the vaccine participants were given in the trial is not found to be effective enough at providing a boost overall, and if a participant's age group become eligible for NHS boost immunisation during 2021/2, then they will receive the approved NHS booster vaccine as part of the trial.
Another part of the trial will involve giving half-doses of certain anti-coronavirus vaccines, to see if the vaccine supply could be made to last for double the number of vaccinations, and also to test whether or not any side-effects are reduced.
The scientists continued:
"By giving a booster dose with these vaccines, we are hoping to ensure that the body produces an immune response that will help provide extra protection against COVID-19 virus infections and disease.
"Because using different COVID-19 vaccines as a booster has never been tested before, we do not know if different vaccine combinations will provide an adequate level of increased protection.
"None of these vaccines contain the live SARS-CoV2 virus and therefore cannot cause the infection."
To read more, and consider taking part, browse to: https://www.covboost.org.uk/participate-brighton

 Funding Available For Tree Planting In Chichester District as National Tree Week Begins
Funding Available For Tree Planting In Chichester District as National Tree Week Begins
 West Sussex Firefighters And Emergency Services Carry Out Training Exercise In Chichester Harbour
West Sussex Firefighters And Emergency Services Carry Out Training Exercise In Chichester Harbour
 Shoreham Lifeboat’s Festive Look Boosted By Family-Run Business
Shoreham Lifeboat’s Festive Look Boosted By Family-Run Business
 Final Touches Being Made To £2m Sussex Wastewater Site Upgrade
Final Touches Being Made To £2m Sussex Wastewater Site Upgrade
 AllSaints Founder To Host Sustainable Fashion Show In Brighton To Raise Money For Homelessness In City
AllSaints Founder To Host Sustainable Fashion Show In Brighton To Raise Money For Homelessness In City
 Chichester Community Warden Helping Fight Against Fraud This Christmas
Chichester Community Warden Helping Fight Against Fraud This Christmas
 Wealden Council Calls For Government Rethink On Winter Fuel Payment
Wealden Council Calls For Government Rethink On Winter Fuel Payment
 Snowy Protest At Lewes County Hall Calls For Fossil Fuel Divestment
Snowy Protest At Lewes County Hall Calls For Fossil Fuel Divestment
 Local MP Tours Firefighters’ Centre In Littlehampton
Local MP Tours Firefighters’ Centre In Littlehampton
 Former Portslade Scout Leader Convicted Of 79 Child Sex Offences
Former Portslade Scout Leader Convicted Of 79 Child Sex Offences